Disparities in Research
Addressing Disparities in Research Informing Personalized Medicine
Research Summary
The current clinical research ecosystem has historically underrepresented racial and ethnic minorities, older adults, women, LGBTQIA+ populations, persons with disabilities, and socioeconomically disadvantaged communities. For these underrepresented biomedical research (UBR) communities, systemic disparities and research participation barriers persist even in disease areas characterized by elevated incidence and mortality counts among understudied patient populations. This significantly limits the potential for better health outcomes.
To better understand and develop strategies to address personalized medicine research disparities, PMC convened more than 60 leaders, including 38 from communities historically underrepresented in biomedical research studies, for a series of discussions about the issue. The group recommended eight strategies that researchers can use to build more inclusive study cohorts. Their recommendations are summarized in the table below.
EMPOWER COMMUNITY ENGAGEMENT IN RESEARCH
Recommendation
Expected Impact
1.) Provide resources for community-based organization (CBO) programs to enhance research participation.
CBOs are trusted entities among many UBR communities. Thoughtful investment and capacity building for CBOs can help build lasting pathways for community participation in research and make it easier to recruit diverse participants.
2.) Empower and provide necessary support to CBOs to deliver training in research competency to their communities and to deliver diversity and cultural competency training to researchers.
When a CBO is given the responsibility to represent a UBR community in the context of research and is directly consulted on educating researchers, it sends a clear message about the needs of the community being a priority for personalized medicine research.
3.) Increase funding for Federally Qualified Rural Health Centers (FQHCs), Urban Indian Health programs, and rural health clinics.
Increased investment in FQHCs and rural health programs,
where clinicians have longstanding relationships and trust with community members, can allow for studies to be designed in ways that benefit the community of interest.
4.) Require a Community Impact Board (CIB) to provide consultation within Institutional Review Board (IRB) deliberations, and include two community representatives on an IRB.
Empowering CIBs within IRBs will allow for potential cultural or ethical concerns to be addressed early, and can help limit bias and avoid informational gaps that can render the results less externally valid for UBR communities.
5.) Foster the recruitment of investigators from diverse backgrounds to conduct personalized medicine research through research sponsor-based initiatives.
Research teams that include leaders with diverse community backgrounds and experiences will be vital to recruiting diverse research participants and assuring development of personalized medicine technologies that are inclusive of patients with a range of health circumstances and experiences.
IMPROVE THE COLLECTION & USE OF INCLUSIVE HEALTH DATA
Recommendation
Expected Impact
6.) Examine and highlight gaps in existing real-world data (RWD) sources, and intentionally collect UBR community data to fill those gaps.
Intentional collection and curation of RWD on patients from UBR communities will help ensure inclusive representation of all patient populations in newly collected and existing evidence used in personalized medicine implementation and outcomes research.
7.) Modify and improve systems to capture and share data on social determinants of health (SDOH) in electronic health records (EHRs).
Availability of standardized SDOH information collected in EHRs can enrich personalized medicine research by ensuring that the unique circumstances of UBR participants are accounted for.
8.) Develop and provide resources for community programs designed to ensure that research information is collected, used, and shared responsibly.
Community transparency regarding the empowerment of research participants to make decisions regarding the collection and use of their own research data will help alleviate community perceptions on the potential for data misuse and build community trust in the personalized medicine research enterprise.
Methodology
The five-step process for developing the above recommendations involved a multi-faceted approach incorporating review of available literature, insights from community leaders and experts working to address challenges to equitable research participation, and input from individuals within or working with UBR communities.
Key to this community-engagement approach was the formation of an iterative engagement with a Health Equity Task Force (HETF) comprising 25 research, data management, and community leaders who are part of or are working directly with underrepresented communities (see Appendix A, below). Members of the HETF shared insights into factors leading to disparities in health data used in the research and development of personalized medicine technologies. Through the Community Network Input process, 63 respondents from within HETF member networks identified priority recommendations, rated their impact and feasibility on a scale of 1-5, and suggested refinements (see Appendix C, below).
A conceptual framework that captures the interplay between policies, social structures, and stakeholders’ behaviors that shape the personalized medicine research ecosystem was also developed based on the initial landscape review. HETF members were encouraged to consider how these domains interconnect to drive accountability in advancing health equity.
The recommendation development process resulted in the selection and refinement of eight final recommendations that underrepresented communities believe are important to operationalize for the improvement of diversity and inclusion in personalized medicine research.
Appendix A: PMC Health Equity Task Force
Name Affiliation Shani Hosten AARP Asraa Alhawli ACCESS Community Health and Research Center Roberta Carlin American Association of Health and Disability Richard Knight, M.B.A. American Association of Kidney Patients Oliver J. Kim, L.L.M., J.D. Asian & Pacific Islander American Health Forum Fornessa T. Randal, M.C.R.P. Asian Health Coalition Linda Goler-Blount Black Women’s Health Imperative Barbara Bierer, M.D. Brigham and Women’s Hospital at the Harvard Medical School Earl D. Fowlkes, Jr. Center for Black Equity Gregory Calip, Pharm.D., M.P.H., Ph.D. Flatiron Health (formerly) Michael O. Minor, Ph.D. H.O.P.E. Health and Human Services Partnership of the National Baptist Convention, USA, Inc. Silas Buchanan Institute for eHealth Equity James W. Lillard, Ph.D., M.B.A. Morehouse School of Medicine Alex J. Carlisle, Ph.D. National Alliance against Disparities in Patient Health Lina Victoria Mata-McMurry, M.D. National Minority Quality Forum Alan Morgan, M.P.A. National Rural Health Association John Molina, M.D., J.D., L.H.D. Native Health Stephen Sodeke, Ph.D. Tuskegee University Martin Mendoza, Ph.D. U.S. National Institutes of Health’s All of Us Research Program, U.S Anna Zink, Ph.D. University of Chicago’s Booth Center for Applied AI Amelie G. Ramirez, Dr.Ph., M.P.H. UT Health San Antonio Kendal K. Whitlock, M.P.H. Walgreens Boots Alliance Kellan E. Baker, Ph.D., M.P.H., M.A. Whitman-Walker Institute Randall C. Morgan, Jr., M.D., M.B.A. W. Montague Cobb/National Medical Association Health Institute KiTani Parker Lemieux, Ph.D. Xavier University of Louisiana Appendix B: HETF One-on-One Discussion Guide
Discussion Goals
One-on-one discussions are key to developing robust draft recommendations aimed at increasing diversity and achieving health equity in personalized medicine research. These conversations will allow our team the opportunity to:- Illuminate best practices from Task Force members in their area of work and expertise.
- Create a safe space for more detailed conversations.
- Inform the development of key themes/topics for small group/breakout discussions (to take place during regularly scheduled task force meetings).
Insights from task force members will be invaluable in the drafting of our set of final recommendations. The questions below are meant to encourage open discussion where thoughtful and creative solutions for increasing diversity and achieving health equity can be explored. Leveraging specific areas of expertise of the Task Force members, we will lean into certain questions and discussions as appropriate. Therefore, all questions listed below may not be asked or addressed to a particular task force member. Additionally, as conversations naturally develop, potential questions and areas of inquiry not listed below may be explored.
Guided Discussion Questions
General themes of questions in the guide center around patient/community engagement, defining diversity, research protocols, and data and technology biases. We anticipate these discussions will generate additional themes and areas for further consideration. Focus group sessions will provide an opportunity to explore additional areas raised during the one-on-one sessions, as well as to delve deeper into the areas covered here.Introductory
- Tell me about your area of expertise.
- How is your work contributing to the advancement of health equity and diversity in research or health care?
- How do you see your work relating to personalized medicine specifically? How do you see your work relating to advancing health equity in personalized medicine?
- The COVID-19 pandemic laid bare the mistrust in research, the medical community, and science broadly. This has been exacerbated by mixed messaging in response to the pandemic by institutions tasked with safeguarding public health. How can personalized medicine be messaged in a way that elevates it above the current fray?
- What continues to motivate you to do this work?
Patient/Community Engagement
- What does patient/community engagement mean to you? What does successful engagement look like for patients in the communities in which participation in personalized medicine research is sought?
- What has community engagement looked like in the course of your work (current or previous)? What lessons do you think could be applied moving forward as it relates to personalized medicine research?
- What are the risks of overstating the potential benefits of personalized medicine research as it relates to closing health disparities for underrepresented communities?
- What do you believe is the biggest misconception regarding participation rates in clinical trials for underrepresented communities? How has this misconception hindered progress of increasing diversity and health equity in personalized medicine trials and what steps can be taken to correct this misconception?
- Participant Advisory Boards (Councils) are tools to ensure diverse stakeholders have a voice in clinical trials. What steps can be taken to ensure participants don’t feel their participation is performative for the purposes of allowing researchers to check the box of “representation?”
- Communicating the value of personalized medicine research is essential and key to any engagement strategy. In your view, what stands in the way of communicating that value to the public and communities? Is it the messenger/messages, the lack of tangible examples of success or other factors?
- Looking at the entire healthcare ecosystem from research, development, approval, and patient care and delivery, prevention strategies, etc. — how do we ensure that personalized medicine is realized and impactful to patients? In your opinion, where do we need to focus? And what needs to happen to support that focus?
Defining Diversity
- Understanding that both race and ethnicity are social constructs, what specific recommendations can be made to researchers to ensure equity and biology are at the center of their clinical research?
- How can issues of intersectionality be elevated in importance in clinical trials?
- If a universal definition of diversity were to be created – what would it be?
Research Protocols
- What protocols can be implemented in the beginning of the study design to increase diversity and equity at the beginning of personalized medicine clinical trials?
- The lack of diversity in clinical trials has been documented extensively. What recommendations or policy prescriptions would you offer to ensure there is accountability in the study design?
- When developing inclusion and exclusion criteria, what best practices can be implemented to ensure diversity and equity remain a focal point in the study design?
- Multinational studies have not increased recruitment of diverse populations, and there is decreasing U.S. based recruitment across pharmaceutical clinical trials. What impact does this have on personalized medicine efforts and what recommendations do you have to address this issue?
- Would mandating diversity in trials as a precursor to FDA filing and approval work? Why or why not?
- Who bears the responsibility for helping ensure that research is representative of the population the treatment or research seeks to benefit?
Data and Technology Biases
- How can data and technology be used to advance personalized medicine research? What works? Where are improvements needed?
- Facets of personalized medicine can still rely on biased health data during initial patient encounters (i.e., collection of biased clinical data that enters the EHR). What policies can be implemented to address this reality?
- Ensuring racial equity in data analysis should be a focal point in personalized medicine research. What problems currently exist in making that a reality and what remedies would you recommend?
- What recommendations do you have to ensure racial equity is factored into the data collection life cycle (i.e., planning, data collection, access, analysis, algorithms/statistical tools) and/or put in place contingencies recognizing data researchers are using or collecting data that may not have adequately factored this in?
- Algorithms play a significant role in our healthcare and in research. How concerned are you about the biases of their creators, whether explicit or implicit, and how that impacts research? As personalized medicine research increasingly relies on big data, what recommendations can be put in place to hold researchers accountable to address this issue moving forward?
- Most inequities in database data probably come from a lack of access to care, and this can lead to biases when using data sources that reflect built in access inequity. How do we account for those biases and remove or minimize them when analyzing data from these sources? Addressing the underlying access inequity is one long-term strategy, but what can we do from a data perspective to account for biases in data that already exist?
Closing Themes
- Are you aware of or support existing recommendations that should be factored into recommendations into this work? Note: Ex. Recommendations from federal agencies/other established recommendations
- What are the most impactful strategies you have seen implemented? Why did they work? What can be improved?
- We have discussed many points and areas where improvement is needed to achieve health equity and diversity in personalized medicine research. As we close out our discussion, are there examples where things are going well? Could any of these examples be elevated to be used for policy recommendations?
- What additional items not covered in our discussions today would you like to see further explored?
Appendix C: Community Network Input by Stakeholder Group
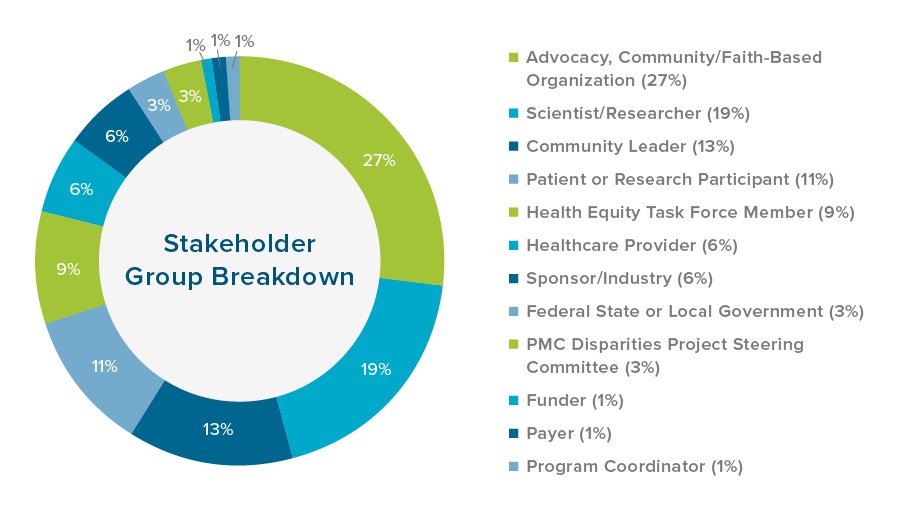
PMC engaged more than 60 leaders, including 38 from historically underrepresented communities, in this research.
This appendix contains information related to community member feedback on the original draft recommendations. Shown in the pie chart below is a breakdown of community members’ stakeholder group and/or profession that responded by percentage:
Appendix D: Community Ratings for Draft Recommendations
This appendix shows the ratings for each of the original draft recommendations considered by community members. Community respondents were asked to select the top three recommendations each felt are most important to be considered for implementation, and to rate them for potential impact and feasibility on a scale of 1 through 5. Results were primarily ranked based on the total number of votes received by community respondents, with feasibility and impact scores averaged to provide context. The top ten recommendations that received the most votes were presented to the HETF and the steering committee. Through an iterative discussion process, some recommendations were combined, and two primary workstreams emerged: community empowerment and intentional data collection and use.
Ranking Draft Recommendations 1 Prioritize Resources for Community-based Organizations 2 Recruit Investigators from Diverse Backgrounds to Lead Studies 3 Empower Community Advisory Board 4 Increase Funding for FQHCs and Rural Health Clinics 5* Intentional Collection and Curation of Race/Ethnicity and SDOH 6 Expand the Use of Real-World Data and Evidence 7 Funding for Organizations to Deliver Diversity and Cultural Competency Training 8* Inclusion of Other Social Characteristics 9 Include Two Community Representatives on an IRB 10 Co-morbidities as a Part of Inclusion Criteria 11 Diversity Requirements in Disease-Specific FDA Guidelines 12 Develop a Community-focused Assessment Tool 13 Requirements by IRBs to Investigators 14 Development Data Systems and Evaluation by Community-based Organizations 15 Create and Implement a Clear Box Label 16 Create an Exception for Compensation for Participation in Research through CMS 17 Expand FDA Clinical Decision Support Guidance 18 Grant FDA Priority Review to Sponsors 19 Compliance with OMB Diversity Definition * Recommendations combined